Curetrax CAR19
CuretraxCAR19 provides treatment-specific support to Care Teams and patients who have received a chimeric antigen receptor (CAR) T cell product targeting the CD19 antigen.
It is well known that there are unique challenges for patients and Care Teams after treatment with CD19 specific CAR T cells. These challenges include the development Cytokine Release Syndrome (CRS), susceptibility to infections and Immune Effector Cell Associated Neurotoxicity (ICAN), all of which have the potential for rapid changes in patient health and wellbeing.
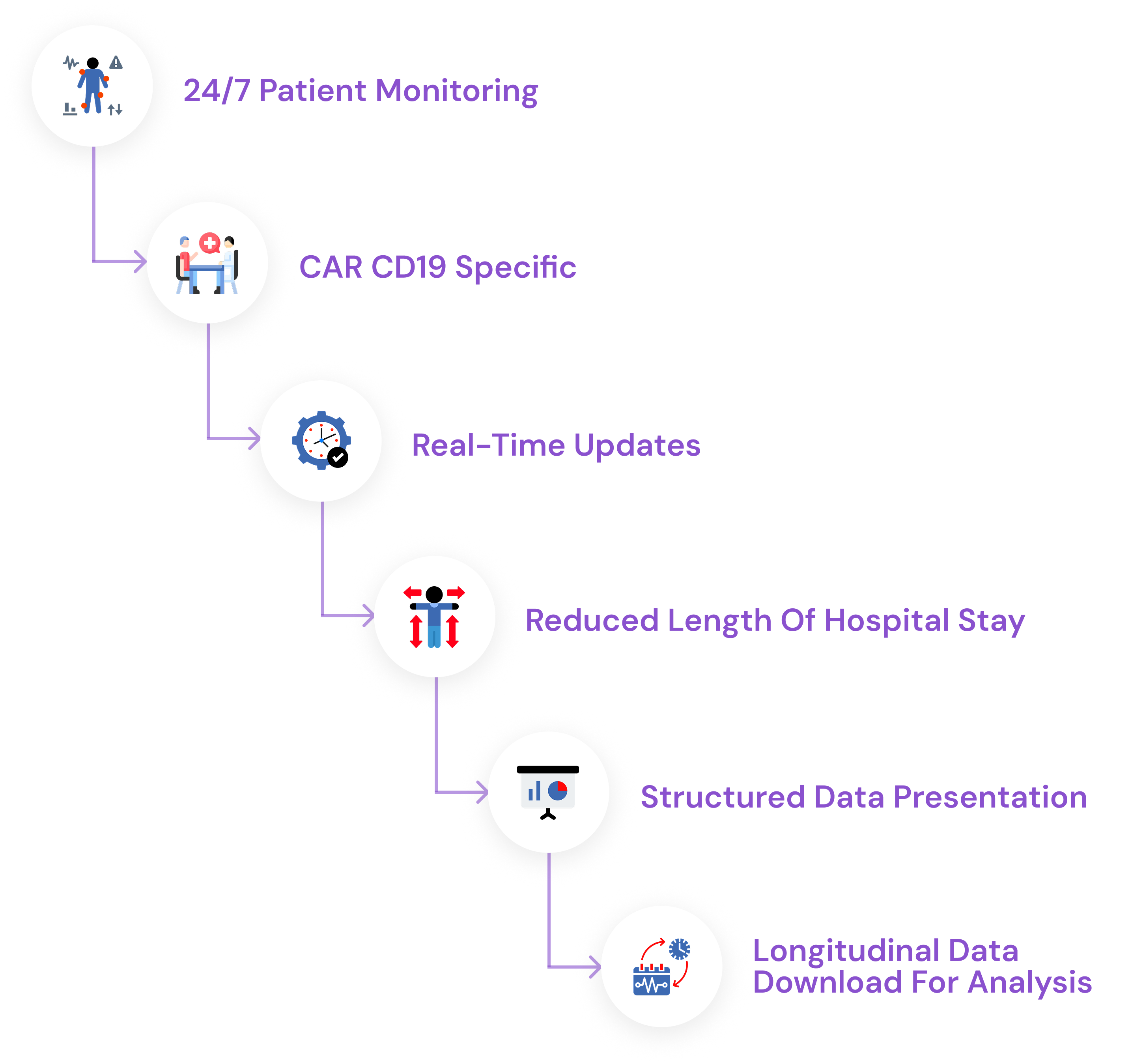
To address these challenges, CuretraxCAR19 provides a platform for Care Teams to securely monitor the health and well-being of their patients 24/7 in real-time.
With CuretraxCAR19, we aim to reduce the lengthy hospital stay and enable outpatient-based treatment, ultimately allowing patients to recover in their more comfortable home environment while freeing much-needed hospital beds and other hospital resources.
About App
To address these challenges, CuretraxCAR provides a platform for Care Teams to securely monitor the health and well-being of their patients 24/7 in real-time.
Key Features

Patient vitals and activity levels such as heart rate, blood oxygen levels and steps are recorded 24/7 through a suitable Garmin wearable. These results are delivered securely in real-time to the supervising Care Team.

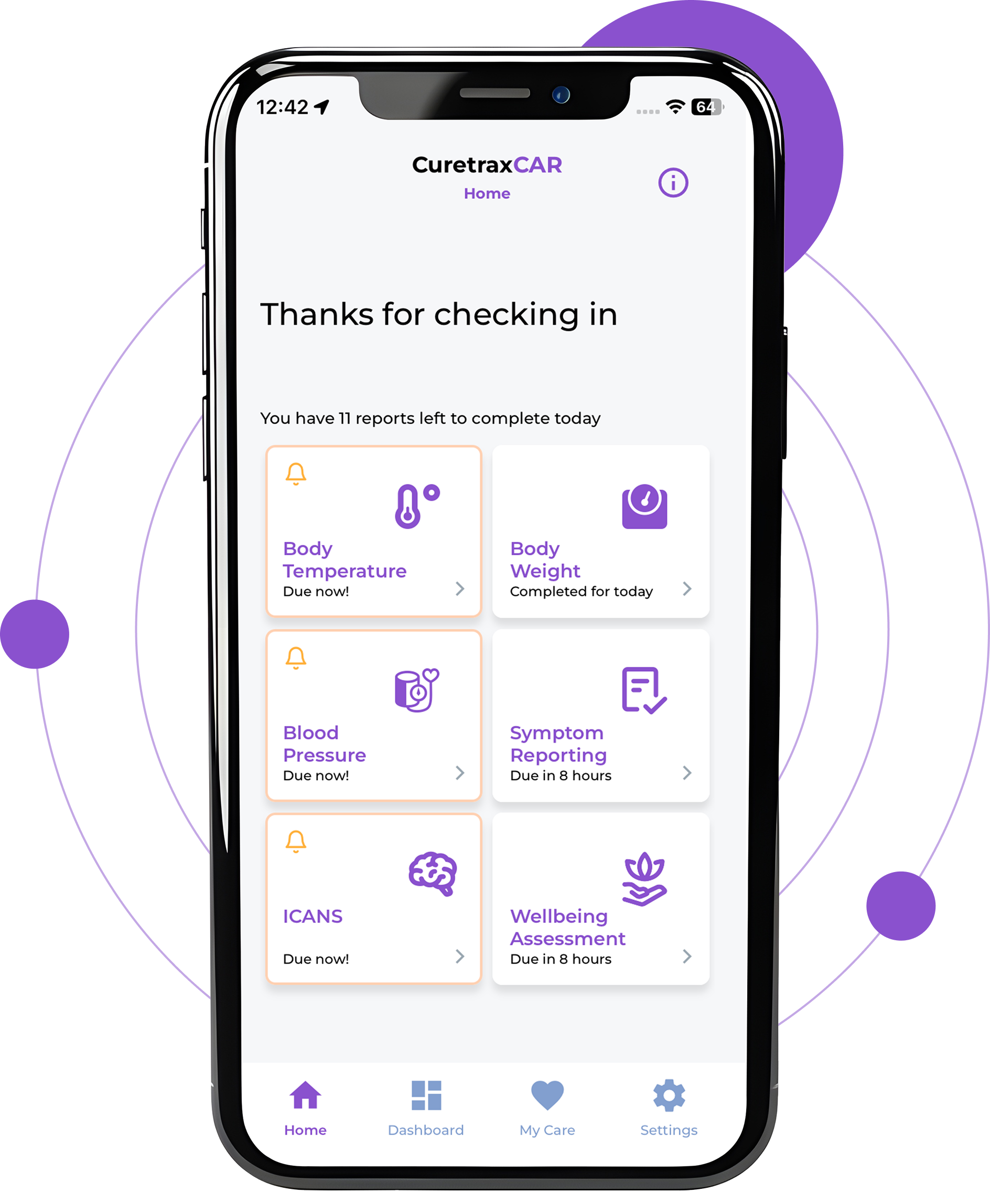
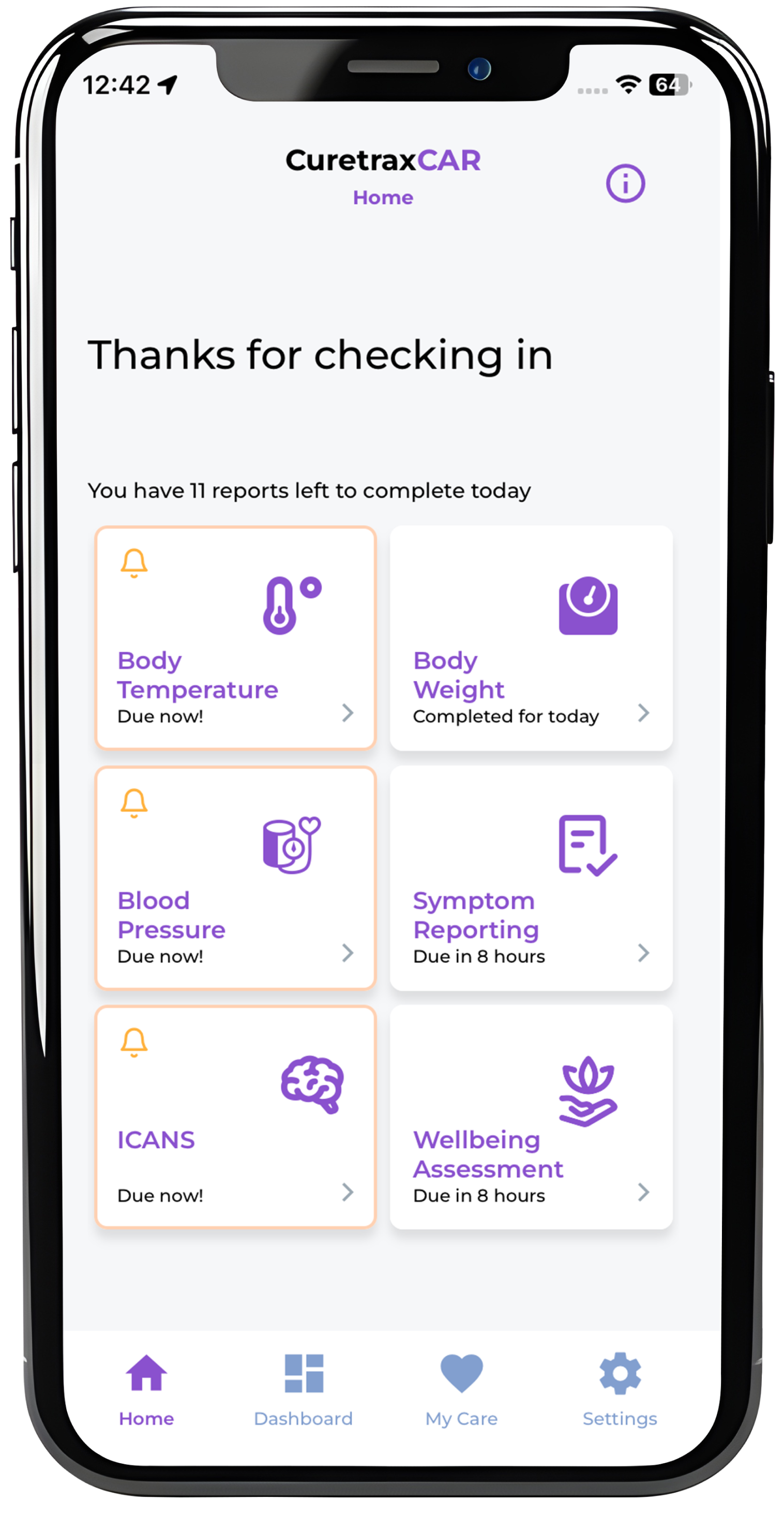
Some health metrics can fluctuate rapidly and are unable to be captured by wearable devices. These metrics, such as blood pressure or body weight, can be reported to Care Teams multiple times during the day through our patient mobile phone application.

CuretraxCAR19 will collect large amounts of patient health data over time. To facilitate easy interpretation and analysis, Care Teams can view neatly formatted health data in their dashboard with the option to view this data over various timeframes.

Keeping track of all the required medications and dosages can be difficult for patients after CAR T cell treatment as they often face a very long daily medication list. Furthermore, often doses need to be adjusted according to blood levels and the patient must be notified. To address this and increase patient compliance, CuretraxCAR19 allows Care Teams to upload patient medication requirements and edit them in real-time and automatically notify the patient about the changes made.

After treatment with CAR T cells targeting the CD19 antigen neurological toxicity can occur. CuretraxCAR19 focuses specifically on assessment of this toxicity by applying standardized tests (for example: Mini-Cog) including a handwriting assessment that can be performed on the screen of a mobile phone or tablet. CuretraxCar19 measures the time to completion for these assessments and displays changes over time to Care Team and visualizes for example in handwriting in comparison to baseline evaluation.

Through our mobile phone application, patients can complete a daily symptom report which provides their Care Team with CAR T cell treatment-specific information about changes in patient health and well-being, such as the development of new symptoms like headache, muscle pain, tremors, nausea, and information on nutrition and bowel movements.

When a patient’s vitals or report responses indicate deviations from normality, the CuretraxCAR platform will send alerts to Care Teams through their dashboard and indicate which metrics should be further investigated.

CuretraxCAR speaks English, French, German and Spanish for now. We are working on including more languages.
Contact Us
We welcome all of those that are interested in learning more about our service to contact us through the form below.

Contact Us
We welcome all of those that are interested in learning more about our service to contact us through the form below.
Learning
About
Technology
Team
Timeline
Healthcare Providers
Products
CuretraxAllo
CuretraxCAR19
© Copyright Curetrax. All Rights Reserved
Terms and privacy
