Timeline
The following timeline provides an overview of the developmental milestones for the Curetrax platform. This timeline will be updated regularly to reflect the current completion progress of Curetrax’s products.
October 2020
Start with an Idea and Find The Right People
After more than 6 months into the COVID-19 pandemic, it became clear that endless unstructured phone consults with patients after stem cell transplantation could become much more informative and efficient if relevant health data could be collected in real-time.
January 2021
Platform Design
Determining the requirements for Curetrax’s first product, CuretraxAllo. Once the requirements for this specific patient population are clearly understood, product design can commence.
January 2021
November 2021
Development of the Mobile and Web Apps Intended for Patients
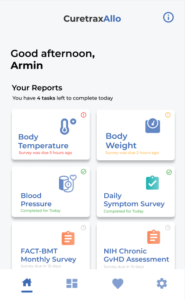
October 2021
Development of the Web Apps Intended for Healthcare Providers

October 2021
July 2022
Development of the Back End Infrastructure
Considering the sensitivity of the data being stored and transmitted within the Curetrax platform, great care and attention is required to ensure patient privacy is maintained. Thus, our back end infrastructure was developed with this being the number one priority.
September 2022
Pilot Study Design
September 2022
November 2022
HIPAA Compliancy Project started
Completion of HIPAA training which ensures Curetrax’s staff and platform are compliant with the regulations specified in HIPAA.
February 2023
Completion of Mobile Application CuretraxAllo
Upon the completion of the development of the Curetrax platform and all required training, Curetrax’s first product, CuretraxAllo, will be formally ready for external testing in the healthcare setting.
February 2023
August 2023
Completion of the Physician´s Dashboard
Full visualistion of the data obtained from the wearable device and the manual symptome reporting to health care takers.
November 2023
Completion of Medication Plan
Interactive Medication Plan added. Healthcare providers can now adjust medications and the patient will be notified. The patient has to acknowledge the change and the healthcare provider will be informed.
November 2023
December 2023
CAR CD19 Application Development started
Our second product development has started. We will build an application designed to allow for home monitoring of patients after CAR T-cell therapy targeting the CD19 antigen. This development will be fully patient centered and in collaboration with patient groups.
May 2024
Funding obtained for 1st CuretraxAllo Clinical Trial
We received an CTTC/LLSC grant funding our first double center trial in Canada in patients after allogeneic stem cell transplantation using our CuretraxAllo application.
May 2024
June 2024
Implementation of a Discussion Tool in CuretraxAllo
In order to be able to discuss patient data among healthcare providers the Physisican´s Dashboard will provide a video and screensharing tool.
August 2024
Completion of CuretraxCAR19 Mobile App
Completion of CuretraxCAR19 mobile phone application for patients after CAR T-cell therapy targting the CD19 antigen.



August 2024
September 2024
Multi-Language Capability
We have now integrated multi-language capabilities allowing patients to choose between English, Francais, Deutsch and Espanol. Presto seguirà l’italiano!

October 2024
Research Ethics Board Application for 1st Clinical Trial Started
We sent out our application to the Research Ethics Board for our first clinical trial.
October 2024
November 2024
Completion of the Curetrax Tower Portal

 The Curetrax Tower Portal brings in multicenter capacity. We can now onboard new centers easily which then can manage themselves autonomously through a center Masteruser. The Masteruser can onboard members of the center, define various roles with different capabilities and manage the team of a center. The Tower has a messenger function that allows for easy communication between centers and the Curetrax team.
The Curetrax Tower Portal brings in multicenter capacity. We can now onboard new centers easily which then can manage themselves autonomously through a center Masteruser. The Masteruser can onboard members of the center, define various roles with different capabilities and manage the team of a center. The Tower has a messenger function that allows for easy communication between centers and the Curetrax team.November 2024
Data Export Function
What happens with the data we collect? Data are stored in our cloud but can be accessed by the center at any time. The data export generates a clean CSV file that can be readily imported into statistical software like R or SPSS for further analysis. Export is possible for individual patients or compiled for all patients over the entire monitoring period.
November 2024
December 2024
Designing CuretraxDoc
Kick off of our 3rd product: Curetrax Doc! This mobile phone application will provide reminders to the health care provider to review patients, will alert health care providers in case of unusual reports and provide a messenger function to connect with the patient if needed. All the functions can be controlled by the health care provider, to prevent overflow with alerts and to be able to sign out when not on service.
